58. Unified Enantioselective Synthesis of 5-Phenylmorphans and cis-Octahydroisoquinolines
Mahgoub, A.; Khamrai, J.; Festus, O.; Altman, R.*
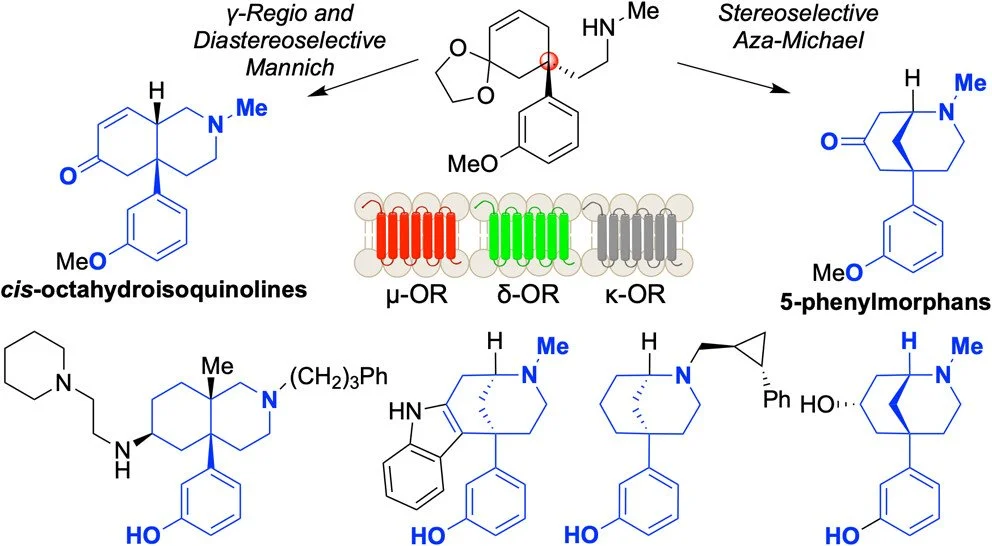
Modifications of morphine have delivered novel opioid scaffolds, such as 5-phenylmorphans and cis-octahydroisoquinolines, that exhibit various pharmacological profiles. To date, these substructures have only been prepared using chiral resolution strategies that require purging of 50% of the material at a late stage of a synthesis. Herein, we disclose the first enantioselective synthesis of both scaffolds. This unified synthesis exploits an enantioselective conjugate addition, followed by conversion of the ester to a common amine intermediate. Subsequently, a diastereoselective aza-Michael reaction generates 5-phenylmorphans, while a γ-regioselective and diastereoselective Mannich reaction generates cis-octahydroisoquinolines.