Publications
-

49. Peroxide-Initiated Hydrophosphination of gem-Difluoroalkenes
Intelli, A. J.; Lee, R. T.; Altman, R. A.*
J. Org. Chem. 2023, 88, 19, 14012–14021.
Synthetic Fluorination Methodology, Fluorinated Alkenes
-

48. Palladium-Catalyzed Dearomatization of Benzothiophenes: Isolation and Functionalization of a Discrete Dearomatized Intermediate
Intelli, A. J.; Pal, M.; Selvaraju, M.; Altman, R. A.*
Synthesis 2023; 55, 21, 3568-3574.
Synthetic Fluorination Methodology, Palladium Catalysis, C—H Functionalization
-

47. A Diselenide Additive Enables Photocatalytic Hydroalkoxylation of gem-Difluoroalkenes
Herrick, R. M.; Abd El-Gaber, M. K.; Rodriguez, L. G. C.; Altman, R. A.*
Chem. Commun. 2023, 59, 5623–5626.
Synthetic Fluorination Methodology, Fluorinated Alkenes
-

46. Photocatalytic Hydrothiolation of gem-Difluoroalkenes
Sorrentino, J. P.; Herrick, R. M.; Abd El-Gaber, M. K.; Abdelazem, A. Z.; Altman, R. A.*
J. Org. Chem. 2022, 87, 16676–16690.
Synthetic Fluorination Methodology, Fluorinated Alkenes
-

45. Cu(II)-Catalyzed Oxidation of gem-Difluoroalkenes Generate α,α-Difluorinated-α-Phenoxyketones
Koley, S.; Cayton, K. T; González-Montiel, G. A.; Yadav, M. R.; Orsi, D. L.; Intelli, A. J; Cheong, P. H.-Y.;* Altman, R. A.*
J. Org. Chem. 2022, 87, 10710–10725.
Synthetic Fluorination Methodology, Fluorinated Alkenes
-

44. Fluoroalkylation of Dextromethorphan Improves CNS Exposure and Metabolic Stability
Sorrentino, J.; Altman, R. A.*
ACS Med. Chem. Lett. 2022, 13, 707–713.
Medicinal Chemistry, Fluorination in Medicinal Chemistry
-

43. Modulating β-Arrestin-2 Recruitment at the δ- and μ-Opioid Receptors
Sharma K. S.; Cassell, R. J.; Meqbil, Y. J.; Su, H.; Blaine, A. T.; Cummins, B. R.; Mores, K. L.; Johnson, D.; van Rijn, R. M.;* Altman, R. A.*
RSC Med. Chem. 2021, 12, 1958–1967.
Medicinal Chemistry, Delta Opioid Receptor, Fluorination in Medicinal Chemistry
-

42. Fluorine-Retentive Strategies for the Functionalization of gem-Difluoroalkenes
Sorrentino, J. P.; Altman, R. A.*
Synthesis 2021, 53, 3935–3950.
Synthetic Fluorination Methodology, Fluorinated Alkenes
-

41. Acid-catalyzed Hydrothiolation of gem-Difluorostyrenes to Access α,α-Difluoroalkylthioethers
Sorrentino, J. P.; Orsi, D. L.; Altman, R. A.*
J. Org. Chem. 2021, 86, 2297–2311.
Synthetic Fluorination Methodology, Fluorinated Alkenes
-

40. Diflunisal Derivatives as Modulators of ACMS Decarboxylase Targeting the Tryptophan-Kynurenine Pathway
Yang, Y.; Borel, T.; de Azambuja, F.; Johnson, D.; Sorrentino, J. P.; Udokwu, C.; Davis, I.; Liu, A.;* Altman, R. A.*
J. Med. Chem. 2021, 64, 797–811.
Medicinal Chemistry, Fluorination in Medicinal Chemistry, Kynurenine Pathway
-

39. Arylation of gem-difluoroalkenes using a Pd/Cu Co-catalytic system that avoids β-fluoride elimination
Yuan, K; Feoktistova, T.; Cheong, P. H.-Y.;* Altman, R. A.*
Chem. Sci. 2021, 12, 1363–1367.
Synthetic Fluorination Methodology, Fluorinated Alkenes
-

38. Cobalt-Catalyzed Selective Unsymmetric Dioxidation of β,β-Difluorostyrenes
Orsi, D. L.; Sorrentino, J. P.; Douglas, J. T.; Altman, R. A.*
J. Org. Chem. 2020, 85, 10451–10465.
Synthetic Fluorination Methodology, Fluorinated Alkenes
-

37. Late-Stage Conversion of a Metabolically Labile Aryl Methyl Ether-Containing Natural Product to Fluoroalkyl Analogues
Sorrentino, J. S.; Ambler, B. R.; Altman, R. A.*
J. Org. Chem. 2020, 85, 5416–5427.
Medicinal Chemistry, Fluorination in Medicinal Chemistry
-

36. Recent Advances in Transition Metal Catalyzed Functionalization of gem-Difluoroalkenes
Koley, S.; Altman, R. A.*
Isr. J. Chem. 2020, 60, 313–339.
Synthetic Fluorination Methodology, Fluorinated Alkenes, Reviews
-

35. Connecting Remote C–H Bond Functionalization and Decarboxylative Coupling Using Simple Amines
de Azambuja, F.; Yang, M.-H.; Feoktistova, T.; Selvaraju, M.; Brueckner, A. C.; Grove, M. A.; Koley, S.; Cheong, P. H.-Y.;* Altman, R. A.*
Nature Chem. 2020, 12, 489–496.
Other Synthetic Methodology, Decarboxylative Coupling
-

34. The Meta-Position of Phe4 in Leu-enkephalin Regulates Potency, Selectivity, Functional Activity, and Signaling Bias at the Delta and Mu Opioid Receptors
Cassell, R. J.; Sharma K. S.; Su, H.; Cummins, B. R.; Cui, H.; Mores, K. L.; Blaine, A. T.; Altman, R. A.;* van Rijn, R. M.*
Medicinal Chemistry, Delta Opioid Receptor
-

33. BBDFA: A Practical Reagent for Trifluoromethylation of Allylic and Benzylic Alcohols on Preparative Scale
Han, C.;* Alabanza, L. M.; Kelly, S. M.; Orsi, D. L.; Gosselin, F.; Altman, R. A.*
Org. Proc. Res. Dev. 2019, 23, 1695–1702.
Synthetic Fluorination Methodology, Decarboxylative Coupling
-

32. Organocatalytic Strategy for Hydrophenolation of Gem-Difluoroalkenes
Orsi, D. L.; Yadav, M. R.; Altman, R. A.*
Tetrahedron 2019, 75, 4325–4336.
Synthetic Fluorination Methodology, Fluorinated Alkenes
-

31. Synthesis of C2-Deutero-L-Tryptophan by Sequential Ir-Catalyzed Reactions
Vallakati, R. K.; Plotnikov, A. T.; Altman, R. A.*
Tetrahedron 2019, 75, 2261–2264.
Other Synthetic Methodology
-

30. Catalytic One-Step Deoxytrifluoromethylation of Alcohols
de Azambuja, F.; Lovrien, S. K.; Ross, P.; Ambler, B. R.; Altman, R. A.*
J. Org. Chem. 2019, 84, 2061–2071.
Synthetic Fluorination Methodology, Decarboxylative Coupling
-
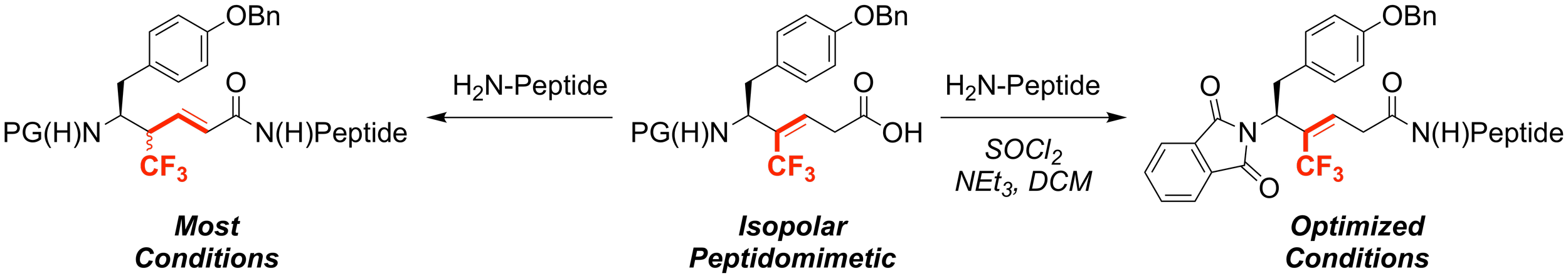
29. Synthesis of Leu-Enkephalin Peptidomimetics Containing Trifluromethylalkenes as Amide Isopolar Mimics
Eeda, V.; Selvaraju, M.; Altman, R. A.*
J. Fluorine Chem. 2019, 218, 90–98.
Medicinal Chemistry, Fluorination in Medicinal Chemistry
-

28. Tyr1-ψ[(Z)CF═CH]-Gly2 Fluorinated Peptidomimetic Improves Distribution and Metabolism Properties of Leu-Enkephalin
Altman, R. A.;* Sharma, K. K.; Rajewski, L. G.; Toren, P. C.; Baltezor, M. J.; Pal, M.; Karad, S. N.
ACS Chem. Neurosci. 2018, 9, 1735–1742.
Medicinal Chemistry, Fluorination in Medicinal Chemistry
-

27. Stepwise O-Atom Transfer in Heme-based Tryptophan Dioxygenase: The Role of Substrate Ammonium in the Epoxide Ring Opening
Shin, I.; Ambler, B. R.; Wherritt, D.; Griffith, W.; Maldonado, A.; Altman, R. A.; Liu, A.*
J. Am. Chem. Soc. 2018, 140, 4372–4379.
Medicinal Chemistry
-

26. Exploiting the Unusual Effects of Fluorine in Methodology
Orsi, D. L.; Altman, R. A.*
Chem. Commun. 2017, 53, 7168–7181.
Synthetic Fluorination Methodology, Reviews
-

25. Base Catalysis Enables Access to α,α-Difluoroalkylthio-ethers
Orsi, D. L.; Easley, B. J.; Lick, A. M.; Altman, R. A.*
Org. Lett. 2017, 19, 1570–1573.
Synthetic Fluorination Methodology, Fluorinated Alkenes
-

24. Synthesis and Opioid Activity of Tyr1–ψ[(Z)CF=CH]–Gly2 and Tyr1–ψ[(S)/(R)-CF3CH–NH]–Gly2 Leu-enkephalin Fluorinated Peptidomimetics
Karad, S. N.; Pal, M.; Crowley, R. S.; Prisinzano, T. E.; Altman, R. A.*
ChemMedChem 2017, 12, 571–576.
Medicinal Chemistry, Fluorination in Medicinal Chemistry
-

23. Metal-catalyzed Decarboxylative Fluoroalkylation Reactions
Ambler, B. R.; Yang, M.-H.; Altman, R. A.*
Synthetic Fluorinated Methodology, Decarboxylative Coupling, Reviews
-

22. Palladium Catalysis Enables Benzylation of α,α-Difluoroketone Enolates
Yang, M.-H.; Hunt, J. R.; Sharifi, N. Altman, R. A.*
Angew. Chem. Int. Ed. 2016, 55, 9080–9083.
Synthetic Fluorination Methodology, Decarboxylative Coupling
-

21. Copper-Catalyzed Synthesis of Trifluoroethylarenes from Benzylic Bromodifluoroacetates
Ambler, B. R.; Zhu, L.; Altman, R. A.*
J. Org. Chem. 2015, 80, 8449–8457.
Synthetic Fluorination Methodology, Decarboxylative Coupling
-

20. Ligand-controlled Regioselective Cu-Catalyzed Trifluoromethylation to Generate Trifluoromethyl Allenes
Ambler, B. R.; Peddi, S.; Altman, R. A.*
Org. Lett. 2015, 17, 2506–2509.
Synthetic Fluorination Methodology, Decarboxylative Coupling
-

19. Ligand-Controlled Regiodivergent Palladium-catalyzed Decarboxylative Allylation Reaction to Access α,α-Difluoroketones
Yang, M.-H.; Orsi, D.; Altman, R. A.*
Angew. Chem. Int. Ed. 2015, 54, 2361–2365.
Synthetic Fluorination Methodology, Decarboxylative Coupling
-

18. Metal-free Trifluoromethylation of Aromatic and Heteroaromatic Aldehydes and Ketones
Qiao, Y.; Si, T.; Yang, M.-H.; Altman, R. A.*
J. Org. Chem. 2014, 79, 7122–7131.
Synthetic Fluorination Methodology, Decarboxylative Coupling
-

17. Copper-catalyzed Conversion of Propargylic Bromodifluoroacetates to Trifluoromethanes
Ambler, B. R.; Peddi, S.; Altman, R. A.*
Synthesis 2014, 46, 1938–1946.
Synthetic Fluorination Methodology, Decarboxylative Coupling
-

16. Decarboxylative Fluorination Strategies for Accessing Medicinally-Relevant Products
Qiao, Y.; Ambler, B. R.; Zhu, L.; Altman, R. A.*
Curr. Top. Med. Chem. 2014, 14, 966–978.
Synthetic Fluorination Methodology, Decarboxylative Coupling, Medicinal Chemistry, Fluorination in Medicinal Chemistry, Reviews
-

15. Copper-Catalyzed Trifluoromethylation of Allylic Bromodifluoroacetic Esters
Ambler, B. R.; Altman, R. A.*
Org. Lett. 2013, 15, 5578–5581.
Synthetic Fluorination Methodology, Decarboxylative Coupling
-

14. Copper-Mediated Deoxygenative Trifluoromethylation of Benzylic Xanthates: Formation of C(sp3)–CF3 from an O-Based Electrophile
Zhu, L.; Liu, S.; Douglas, J. T.; Altman, R. A.*
Chem. Eur. J. 2013, 19, 12800–12805.
Synthetic Fluorination Methodology
-

13. Preparation of Fluoroalkenes via the Shapiro Reaction: Direct Access to Fluorinated Peptidomimetics
Yang, M. H.; Matikonda, S. S.; Altman, R. A.*
Org. Lett. 2013, 15, 3894–3897.
Synthetic Fluorination Methodology, Fluorinated Alkenes
-
Graduate and Postdoctoral Careers
12) Altman, R. A.; Nilsson, B. N.; Overman, L. E.;* Read de Alaniz, J.; Rohde, J. M.; Taupin, V. “Synthesis of (+)-Nankakurines A and B and (±)-5-epi-Nankakurine A” J. Org. Chem. 2010, 75, 7519–7534.
11) Taylor, A. M.; Altman, R. A.; Buchwald, S. L.* “Palladium-catalyzed Enantioselective α-Arylation and α-Vinylation of Oxindoles Facilitated by an Axially Chiral P-Stereogenic Ligand" J. Am. Chem. Soc. 2009, 131, 9900–9901.
10) Altman, R. A.; Hyde, A. M.; Huang, X.; Buchwald, S. L.* “Orthogonal Pd- and Cu-Based Catalyst Systems for the C- and N-Arylation of Oxindoles” J. Am. Chem. Soc. 2008, 130, 9613–9620.
9) Altman, R. A.; Anderson, K. W.; Buchwald, S. L.* “Pyrrole 2-Carboxylic Acid as a Ligand for the Cu-Catalyzed Reactions of Primary Anilines with Aryl Halides” J. Org. Chem. 2008, 73, 5167–5169.
8) Altman, R. A.; Shafir, A.; Choi, A. C.; Lichtor, P. A.; Buchwald, S. L.* “An Improved Cu-Based Catalyst System for the Reactions of Alcohols with Aryl Halides” J. Org. Chem. 2008, 73, 284–286.
7) Altman, R. A.; Buchwald, S. L.* “Pd-Catalyzed Suzuki-Miyaura Reactions of Aryl Halides Using Bulky Biaryl-monophosphine Ligands” Nature Prot. 2007, 2, 3115–3121.
6) Altman, R. A.; Buchwald, S. L.* “Pd-Catalyzed Amination Reactions of Aryl Halides Using Bulky Biarylmono-phosphine Ligands” Nature Prot. 2007, 2, 2881–2887.
5) Altman, R. A.; Buchwald, S. L.* “Cu-Catalyzed Goldberg and Ullmann Reactions of Aryl Halides Using Diamine and Diketone Ligands” Nat Prot. 2007, 2, 2474–2479.
4) Altman, R. A.; Koval, E. D.; Buchwald, S. L.* “Copper-Catalyzed N-Arylation of Imidazoles and Benzimidazoles” J. Org. Chem. 2007, 72, 6190–6199.
3) Altman, R. A.; Buchwald, S. L.* “Cu-Catalyzed N- and O-Arylation of 2-, 3- and 4-Hydroxypyridines and Hydroxyquinolines” Org. Lett. 2007, 9, 643–646.
2) Anderson, K. W.; Tundel, R. E.; Ikawa, T.; Altman, R. A.; Buchwald, S. L.* “Monodentate Phosphines Provide Highly Active Catalysts for Pd-Catalyzed C–N Bond-Forming Reactions of Heteroaromatic Halides/Amines and (H)N-Heterocycles” Angew. Chem. Int. Ed. 2006, 45, 6523–6527.
1) Altman, R. A.; Buchwald, S. L.* “4,7-Dimethoxy-1,10-phenanthroline: An Excellent Ligand for the Cu-Catalyzed N-Arylation of Imidazoles” Org. Lett. 2006, 8, 2779–2782.
-
Book Chapters
2) de Azambuja, F.; Altman, R. A.* “NFSI and Its Analogs Fluorination for Preparing Alkenyl Fluorides (Transition-Metal Free)” in Synthetic Organofluorine Chemistry. Fluorination. Hu, J., Umemoto, T. Eds. Springer Nature Singapore Pte Ltd.: Singapore, 2017; Volume 1, Section 20.
1) de Azambuja, F.; Altman, R. A.* “SelectFluor and Its Analogs Fluorination for Preparing Alkenyl Fluorides (Transition-metal free)” in Synthetic Organofluorine Chemistry. Fluorination. Hu, J., Umemoto, T. Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2017; pp. Volume 1, Section 19.
-
Minor Works
12) Altman, R. A.* “In This Issue” ACS Med. Chem. Lett. 2023, 14, 1132–1133
11) Altman, R. A.* “In This Issue” ACS Med. Chem. Lett. 2022, 13, 1827-1828.
10) Yang, M.-H.; Altman, R. A. “News and Views: β-Elimination Rules for Pd” Nat. Synth. 2022, 1, 753–754.
9) Adams, A; Altman, R.; Brai, A.; Golden, J.; La Regina, G.; Li, Z.; Moore, T.; Pomerantz, W.; Rajapaksa, N. “An Innovation 10 Years in the Making: The Stories in the Pages of ACS Medicinal Chemistry Letters” ACS Med. Chem. Lett. 2022, 13, 540-545.
8) Altman, R.A.* “In This Issue” ACS Med. Chem. Lett. 2022, 13, 3, 328-329
7) Altman, R. A.* “In This Issue” ACS Med. Chem. Lett. 2021, 12, 670–671.
6) Altman, R. A.* “In This Issue” ACS Med. Chem. Lett. 2020, 11, 1783–1784.
5) Altman, R. A.* “In This Issue” ACS Med. Chem. Lett. 2020, 11, 1661–1662.
4) Altman, R. A.* “In This Issue” ACS Med. Chem. Lett. 2019, 10, 836–837.
3) Altman, R. A.* “Editorial: Fluorination and Fluorinated Compounds in Medicinal Chemistry” Curr. Top. Med. Chem. 2014, 7, 837–839.
2) Zhu, L.; Altman, R. A.* “3,4,7,8-Tetramethyl-1,10-Phenanthroline (tmphen)” Electronic Encyclopedia of Reagents Organic Synthesis [Online]; John Wiley & Sons, Ltd., Published Online April 22, 2013.
1) Altman, R. A.* “1,10-Phenanthroline, 1,10-Dimethoxy-” Electronic Encyclopedia of Reagents Organic Synthesis [Online]; John Wiley & Sons, Ltd. Published Online September 15, 2008.