Medicinal and Biological Chemistry
Our synthetic chemistry expertise enables us to actively collaborate with pharmacological and biochemical experts to develop biological probes and therapeutic candidates for treating a range of diseases. In these collaborations, we are responsible for (a) designing new analogs using structure-guided design, (b) synthesis of new analogs of lead molecules, (c) using available co-crystal structures to conduct virtual screens of large compound libraries, (d) conducting in vitro physicochemical characterization of analogs, (e) conducting and/or coordinating in vitro stability, metabolism and distribution assays, (f) delivering sufficient quantities of top performing analogs to collaborators for in vivo studies.
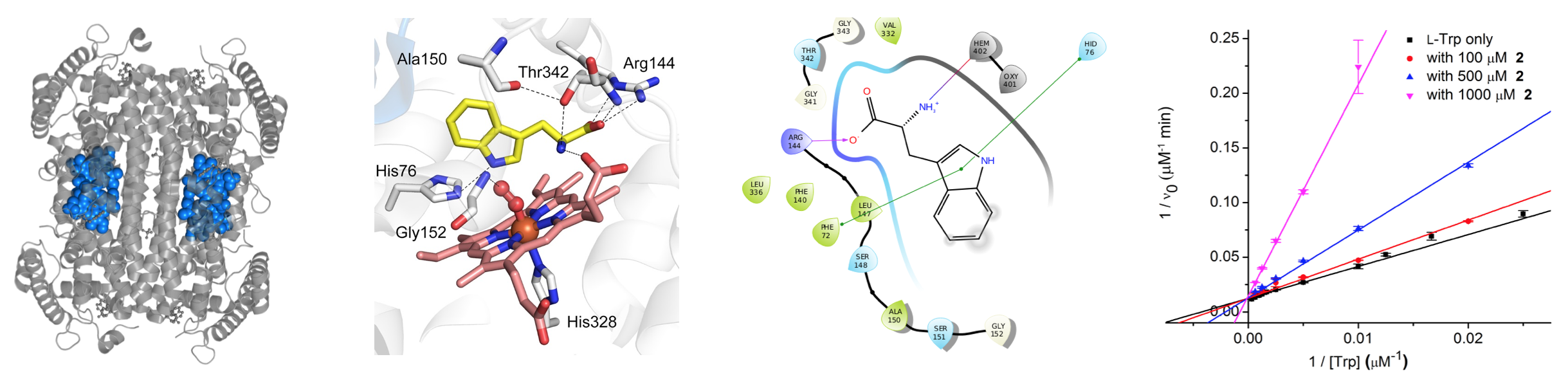
The kynurenine pathway (KP) regulates tryptophan metabolism and generates many modulatory biomolecules that in turn directly correlate to affect various aspects of neurotransmission, neurotoxicity, neuroprotection, inflammation, and other immunological functions. Further, dysregulation of this pathway directly correlates to many disease states, including neurological disorders, infectious diseases, and cancer, thus making small molecule modulators of the KP critical for understanding the diseases states, and for providing potential therapies.
Within this area, we work collaboratively to develop small molecule probes for studying and modulating enzymes in the kynurenine pathway. In some cases, these probes are used to study unique aspects of KP enzymology, while other efforts aim to develop small-molecule probes for modulating in vitro and in vivo models of various disease states. Long-term, these biological probes might serve as leads for downstream medicinal chemistry optimization. To support this project, we actively collaborate with the research group of Prof. Aimin Liu.
The delta opioid receptor regulates several activities within the central nervous system (CNS), and this target is particularly interesting for treating chronic pain and migraines, depression, anxiety, and alcohol use disorder. Despite these potential therapeutic utilities, delta agonists have been historically limited by adverse effects that limit clinical use, which are linked to ligands that signal through beta-arrestin 2 proteins.
To realize the therapeutic potential of this target, we develop delta-selective ligands with novel signaling profiles, specifically agonists that selectively activate therapeutically relevant G-protein coupling pathways, while avoiding activation of the beta-arrestin 2 pathway related to adverse effects. In this area, we are interested in exploring both peptide-based and synthetic agonists of the delta receptor.