60. On the Metabolic Stability of Fluorinated Small Molecules: A Physical Organic Chemistry Perspective
Bhattarai, P. B.; Trombley, T. A.; Altman, R. A.* ChemRxiv, https://doi.org/10.26434/chemrxiv-2025-mtqbs
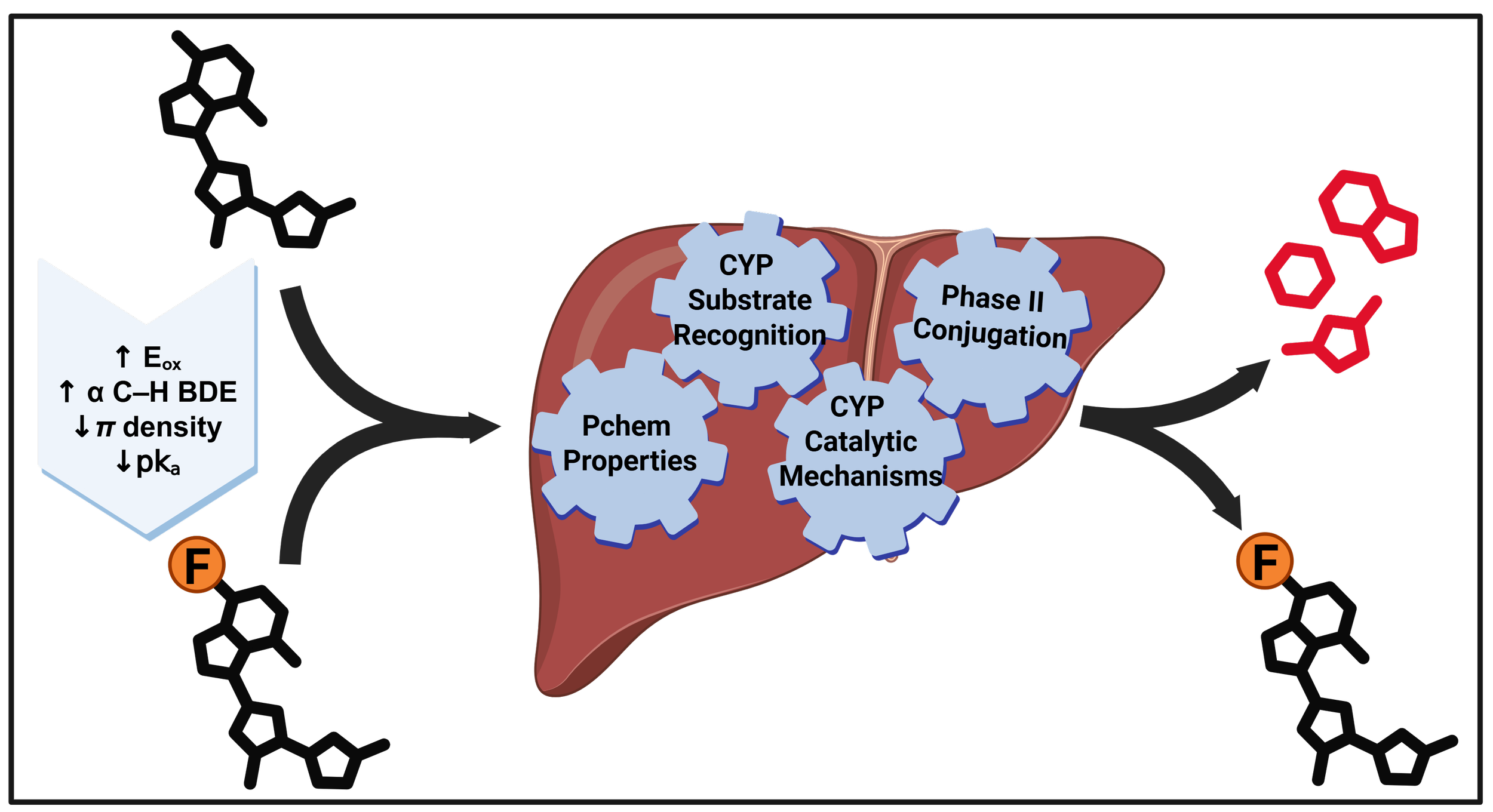
The incorporation of fluorine and fluorinated motifs into medicinally relevant scaffolds can improve the metabolic and pharmacokinetic properties (DMPK) of drug molecules. Typically, this phenomenon is attributed to differences in C–H vs. C–F bond strengths, though such an explanation ignores accepted mechanisms of drug metabolism and how fluorine and fluorinated substituents perturb physicochemical properties of small molecules. Instead of relying on bond strengths, this perspective will review established mechanisms of drug metabolism and provide mechanistic rationale for the improved metabolic profiles achieved by fluorinating drug candidates. This discussion will connect observed trends in structure-metabolism-relationships with known mechanistic features of relevant drug-metabolizing enzymes, with the ultimate goal of improving medicinal chemistry decision-making.